Dive into the fascinating world of chemical equations with our comprehensive Chemical Equations Gizmo Answer Key. Embark on a journey of discovery as we unravel the mysteries of chemical reactions, equipping you with the knowledge to navigate this captivating realm.
Our answer key provides an in-depth exploration of chemical equations, their types, and their significance in predicting reaction products and performing stoichiometry calculations. With clear explanations and engaging examples, we’ll empower you to master this essential aspect of chemistry.
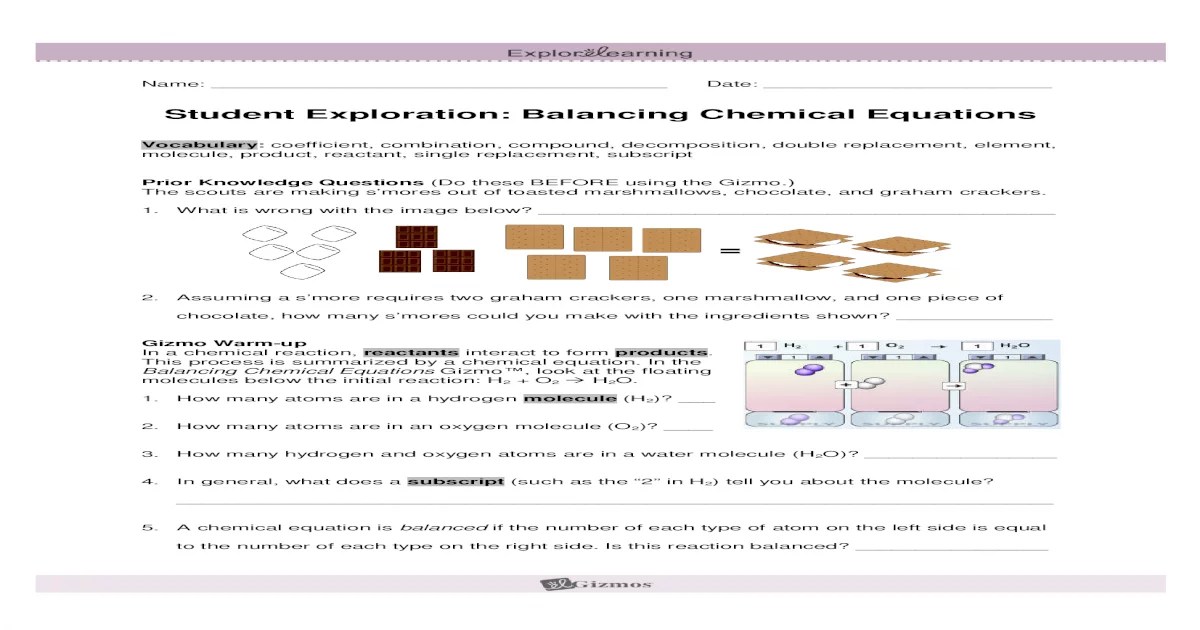
Balancing Chemical Equations
Balancing chemical equations involves adjusting the stoichiometric coefficients in front of each chemical formula to ensure that the number of atoms of each element is the same on both sides of the equation. This process ensures that the law of conservation of mass is upheld, which states that matter cannot be created or destroyed during a chemical reaction.An
unbalanced chemical equation is one in which the number of atoms of each element is not the same on both sides of the equation. A balanced chemical equation is one in which the number of atoms of each element is the same on both sides of the equation.To
balance a chemical equation, the following steps can be followed:
- Start by identifying the unbalanced equation.
- Count the number of atoms of each element on both sides of the equation.
- Adjust the stoichiometric coefficients in front of each chemical formula until the number of atoms of each element is the same on both sides of the equation.
Types of Chemical Equations
Chemical equations are symbolic representations of chemical reactions that provide information about the reactants, products, and the stoichiometry of the reaction. Different types of chemical equations exist, each with its own characteristics and applications.
Synthesis Reactions
Synthesis reactions are chemical reactions in which two or more substances combine to form a single product. These reactions are characterized by the joining of two or more molecules or atoms to form a more complex molecule.
- Example:2H 2+ O 2→ 2H 2O
Decomposition Reactions
Decomposition reactions are chemical reactions in which a single compound breaks down into two or more simpler substances. These reactions are characterized by the splitting of a molecule into smaller molecules or atoms.
- Example:2H 2O → 2H 2+ O 2
Single Displacement Reactions, Chemical equations gizmo answer key
Single displacement reactions are chemical reactions in which one element replaces another element in a compound. These reactions are characterized by the replacement of one element by another element in a compound.
- Example:Fe + 2HCl → FeCl 2+ H 2
Double Displacement Reactions
Double displacement reactions are chemical reactions in which two compounds exchange ions to form two new compounds. These reactions are characterized by the exchange of ions between two compounds to form two new compounds.
- Example:NaCl + AgNO 3→ AgCl + NaNO 3
Predicting Products of Reactions
Chemical equations are essential tools for predicting the products of reactions. They provide a symbolic representation of the reactants and products involved in a chemical reaction, along with the stoichiometric ratios between them. By analyzing the coefficients in an equation, we can determine the mole ratios of reactants and products, which allows us to predict the amounts of substances that will be produced or consumed in a reaction.
Using Coefficients to Determine Mole Ratios
The coefficients in a chemical equation represent the number of moles of each reactant and product involved in the reaction. For example, in the equation:“`
H2 + O2 → 2H2O
“`The coefficients indicate that 2 moles of hydrogen (H2) react with 1 mole of oxygen (O2) to produce 2 moles of water (H2O). This means that for every 2 moles of hydrogen used, 1 mole of oxygen is required, and 2 moles of water will be produced.
Predicting Products Using Chemical Equations
To predict the products of a reaction using a chemical equation, follow these steps:
- Identify the reactants and products in the equation.
- Determine the mole ratios of the reactants and products using the coefficients.
- Use the mole ratios to calculate the amounts of reactants and products that will be involved in the reaction.
For example, if we want to predict the products of the reaction between 4 moles of hydrogen and 2 moles of oxygen, we can use the following steps:Identify the reactants (H2 and O2) and products (H2O).
2. Determine the mole ratios
2 moles H2 : 1 mole O2 : 2 moles H2O.
3. Calculate the amounts of reactants and products
Hydrogen
4 moles H2 ÷ 2 moles H2/1 mole O2 = 2 moles O2 required
Oxygen
2 moles O2 ÷ 1 mole O2/2 moles H2O = 4 moles H2O producedTherefore, the reaction between 4 moles of hydrogen and 2 moles of oxygen will produce 4 moles of water.
Stoichiometry Calculations
Stoichiometry is the study of the quantitative relationships between reactants and products in chemical reactions. It involves using balanced chemical equations to calculate the amounts of reactants and products involved in a reaction.
Stoichiometry has various applications in chemistry, including predicting the products of reactions, determining the limiting reactant in a reaction, and calculating the yield of a reaction.
Using Chemical Equations for Stoichiometry Calculations
Balanced chemical equations provide the mole ratios of reactants and products. These ratios can be used to calculate the amount of one substance that will react with or produce a given amount of another substance.
For example, the balanced equation for the combustion of methane is:
CH4+ 2O 2→ CO 2+ 2H 2O
This equation tells us that 1 mole of methane reacts with 2 moles of oxygen to produce 1 mole of carbon dioxide and 2 moles of water.
Stoichiometry Calculations Involving Mass
Stoichiometry calculations can be used to determine the mass of a reactant or product if the mass of another reactant or product is known. To do this, we use the molar mass of the substances involved.
For example, if we know that 10.0 g of methane reacts, we can calculate the mass of carbon dioxide produced using the following steps:
- Convert the mass of methane to moles: 10.0 g CH4× (1 mol CH 4/ 16.0 g CH 4) = 0.625 mol CH 4
- Use the mole ratio from the balanced equation to convert moles of methane to moles of carbon dioxide: 0.625 mol CH 4× (1 mol CO 2/ 1 mol CH 4) = 0.625 mol CO 2
- Convert the moles of carbon dioxide to mass: 0.625 mol CO 2× (44.0 g CO 2/ 1 mol CO 2) = 27.5 g CO 2
Stoichiometry Calculations Involving Moles
Stoichiometry calculations can also be used to determine the number of moles of a reactant or product if the number of moles of another reactant or product is known.
For example, if we know that 0.50 mol of oxygen reacts, we can calculate the number of moles of methane produced using the following steps:
- Use the mole ratio from the balanced equation to convert moles of oxygen to moles of methane: 0.50 mol O2× (1 mol CH 4/ 2 mol O 2) = 0.25 mol CH 4
Stoichiometry Calculations Involving Volume
Stoichiometry calculations can also be used to determine the volume of a gas if the volume of another gas is known. To do this, we use the ideal gas law:
PV = nRT
Where:
- P is the pressure (in atm)
- V is the volume (in L)
- n is the number of moles
- R is the ideal gas constant (0.0821 L⋅atm/(mol⋅K))
- T is the temperature (in K)
For example, if we know that 1.0 L of oxygen at STP (273 K and 1 atm) reacts, we can calculate the volume of carbon dioxide produced using the following steps:
- Convert the volume of oxygen to moles: 1.0 L O2× (1 mol O 2/ 22.4 L O 2) = 0.045 mol O 2
- Use the mole ratio from the balanced equation to convert moles of oxygen to moles of carbon dioxide: 0.045 mol O 2× (1 mol CO 2/ 2 mol O 2) = 0.0225 mol CO 2
- Convert the moles of carbon dioxide to volume: 0.0225 mol CO 2× (22.4 L CO 2/ 1 mol CO 2) = 0.504 L CO 2
Solution Stoichiometry
Solution stoichiometry deals with the quantitative relationships between reactants and products in chemical reactions that occur in solutions. It involves calculations based on the concentrations, volumes, and masses of the reactants and products.
Using chemical equations, solution stoichiometry allows us to determine the amount of reactants or products needed or produced in a reaction. This knowledge is crucial in various applications, including analytical chemistry, environmental monitoring, and industrial processes.
Concentration Calculations
Concentration, typically expressed in molarity (M), is a measure of the amount of solute dissolved in a solvent. In solution stoichiometry, we can use chemical equations to calculate the concentration of a reactant or product, given the concentration and volume of the other.
Molarity (M) = moles of solute / liters of solution
Volume Calculations
Volume measurements are essential in solution stoichiometry. We can use chemical equations to determine the volume of a reactant or product needed or produced, given the volume and concentration of the other.
Volume (L) = moles of solute / concentration (M)
Mass Calculations
Mass calculations in solution stoichiometry involve determining the mass of a reactant or product, given the mass and molar mass of the other. Molar mass is the mass of one mole of a substance.
Mass (g) = moles of solute × molar mass (g/mol)
FAQ Guide: Chemical Equations Gizmo Answer Key
What is the purpose of balancing chemical equations?
Balancing chemical equations ensures that the number of atoms of each element on the reactants’ side equals the number of atoms of that element on the products’ side. This reflects the law of conservation of mass, which states that matter cannot be created or destroyed during a chemical reaction.
How do I determine the coefficients in a balanced chemical equation?
Coefficients in a balanced chemical equation are adjusted until the number of atoms of each element on both sides of the equation is equal. This can be achieved through trial and error or by using algebraic methods.
What is the significance of stoichiometry in chemical reactions?
Stoichiometry allows us to determine the quantitative relationships between reactants and products in a chemical reaction. By using the coefficients in a balanced chemical equation, we can calculate the exact amounts of reactants and products involved in the reaction.