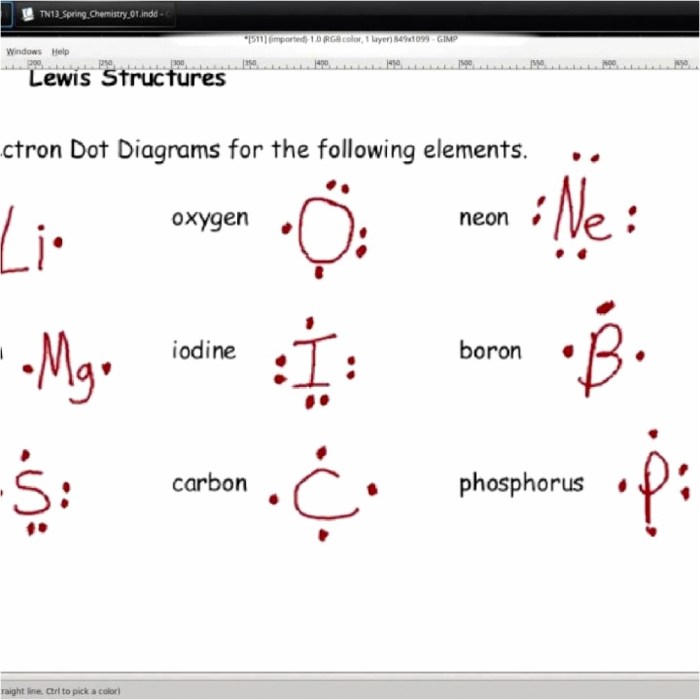
Embark on a captivating journey into the realm of atomic structure with our comprehensive Lewis Structure of Atoms Worksheet. This educational resource unravels the secrets of Lewis structures, empowering you to visualize and understand the fundamental building blocks of our universe.
Delve into the fascinating world of chemical bonding as we explore the concepts of valence electrons, the octet rule, and the exceptions that challenge our understanding. Discover the significance of resonance structures and delve into the intricacies of formal charges, unlocking a deeper comprehension of atomic interactions.
Lewis Structure Fundamentals
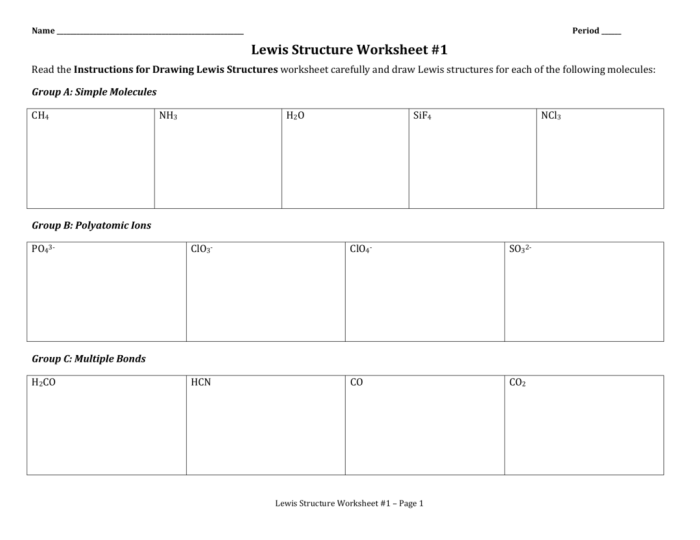
Lewis structures are a representation of the electron configuration of atoms and molecules, showing the arrangement of valence electrons around the atomic nuclei. They are used to predict the bonding behavior and properties of elements and compounds.
Lewis structures follow the octet rule, which states that atoms are most stable when they have eight valence electrons. For example, the Lewis structure of hydrogen (H) is a single dot, representing its one valence electron, while the Lewis structure of oxygen (O) is two dots with a double bond, representing its two valence electrons and the double bond it forms with itself.
Creating Lewis Structures
To create a Lewis structure, follow these steps:
- Determine the number of valence electrons in the atom.
- Place the electrons around the atomic nucleus, following the octet rule.
- If the atom has more than four valence electrons, use double or triple bonds to connect the electrons to the nucleus.
For example, the Lewis structure of nitrogen (N) is a triple bond with two lone pairs, representing its five valence electrons.
Exceptions to the Octet Rule, Lewis structure of atoms worksheet
Some atoms do not follow the octet rule. These exceptions include:
- Atoms with incomplete octets: These atoms have less than eight valence electrons, such as hydrogen (H) and helium (He).
- Atoms with expanded octets: These atoms have more than eight valence electrons, such as sulfur (S) and phosphorus (P).
For example, the Lewis structure of boron (B) has an incomplete octet with only six valence electrons.
Resonance Structures
Resonance structures are different Lewis structures that represent the same molecule or ion. They are used when the electrons in a molecule or ion are delocalized, meaning they are not confined to a single atom.
For example, the Lewis structure of ozone (O3) has two resonance structures, one with a double bond between the central oxygen atom and one of the terminal oxygen atoms, and the other with a double bond between the central oxygen atom and the other terminal oxygen atom.
Formal Charges
Formal charges are a way of calculating the charge of an atom in a molecule or ion. They are used to determine the stability of a molecule or ion.
To calculate the formal charge of an atom, use the following equation:
Formal charge = Valence electrons – Non-bonding electrons – 1/2 Bonding electrons
For example, the formal charge of the central oxygen atom in ozone is zero.
Q&A: Lewis Structure Of Atoms Worksheet
What is the significance of Lewis structures?
Lewis structures provide a visual representation of the arrangement of electrons around atoms, enabling us to understand chemical bonding and predict molecular properties.
How do I determine the number of valence electrons for an atom?
The number of valence electrons is equal to the group number of the element on the periodic table.
What are the exceptions to the octet rule?
Incomplete octets occur when an atom has less than eight valence electrons, while expanded octets occur when an atom has more than eight valence electrons.