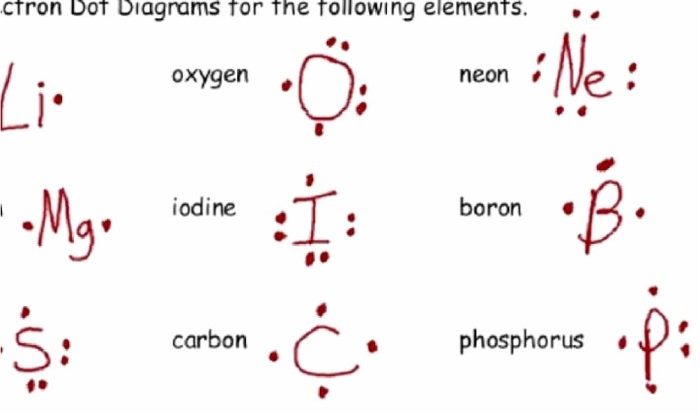
Ionic formula writing worksheet answers provide a structured and systematic approach to understanding the principles of ionic compound formation and formula writing. This guide explores the basics of ionic compounds, the rules for writing their formulas, and the steps involved in answering worksheet questions, empowering students to master this essential chemistry concept.
The content of the second paragraph that provides descriptive and clear information about the topic
Ionic Formula Writing Worksheet Answers
This worksheet provides practice in writing ionic formulas for ionic compounds. Ionic compounds are formed when a metal loses one or more electrons to a nonmetal. The metal becomes a positively charged ion, called a cation, and the nonmetal becomes a negatively charged ion, called an anion.
The charges of the ions must balance each other in order for the compound to be neutral.
To write the ionic formula for an ionic compound, first write the symbol for the metal, followed by the symbol for the nonmetal. Then, balance the charges of the ions by using subscripts. For example, the ionic formula for sodium chloride is NaCl.
Sodium has a charge of +1 and chlorine has a charge of -1, so the subscripts balance the charges.
Worksheet Analysis, Ionic formula writing worksheet answers
The ionic formula writing worksheet includes a variety of questions and exercises. The questions ask students to write the ionic formulas for various ionic compounds. The exercises ask students to identify the ions that make up an ionic compound and to balance the charges of the ions.
The worksheet is appropriate for students who have a basic understanding of ionic bonding. The questions and exercises are clear and concise, and the instructions are easy to follow.
Answer Key Creation
The answer key for the worksheet provides the correct ionic formulas for all of the questions and exercises. The answer key also includes step-by-step instructions for writing ionic formulas.
To write the ionic formula for an ionic compound, follow these steps:
- Write the symbol for the metal.
- Write the symbol for the nonmetal.
- Balance the charges of the ions by using subscripts.
Student Learning Objectives
By completing the ionic formula writing worksheet, students will be able to:
- Define ionic compounds and explain how they are formed.
- Write the ionic formulas for various ionic compounds.
- Identify the ions that make up an ionic compound.
- Balance the charges of the ions in an ionic compound.
The worksheet aligns with the following curriculum standards:
- National Science Education Standards (NSES): Physical Science, Standard B: Properties and Changes of Properties in Matter
- Next Generation Science Standards (NGSS): MS-PS1-2: Matter and Its Interactions
Classroom Implementation
The ionic formula writing worksheet can be used in a variety of ways in the classroom. It can be used as a review activity, a homework assignment, or a quiz. The worksheet can also be used as a starting point for a discussion on ionic bonding.
When using the worksheet in the classroom, it is important to differentiate for students with different learning needs. For example, students who are struggling with the concept of ionic bonding may need more support. This support can be provided by providing them with additional resources, such as a textbook or a website.
Students who are advanced may be able to complete the worksheet independently.
Extensions and Enrichment
The ionic formula writing worksheet can be extended in a variety of ways. For example, students can be asked to research the properties of ionic compounds. They can also be asked to design and build a model of an ionic compound.
Here are some additional ideas for extending the learning beyond the worksheet:
- Have students research the different types of ionic compounds.
- Have students create a poster that explains how ionic compounds are formed.
- Have students build a model of an ionic compound using everyday materials.
- Have students write a report on the importance of ionic compounds in everyday life.
Q&A
What are ionic compounds?
Ionic compounds are formed when a metal loses one or more electrons to a nonmetal, resulting in the formation of positively charged ions (cations) and negatively charged ions (anions) that are held together by electrostatic attraction.
How do I write the formula for an ionic compound?
To write the formula for an ionic compound, you must balance the charges of the ions involved. The subscripts in the formula represent the number of each type of ion needed to achieve a neutral compound.
What is the purpose of an ionic formula writing worksheet?
Ionic formula writing worksheets provide practice in applying the rules for writing ionic formulas and reinforce the concepts of ionic compound formation and charge balance.