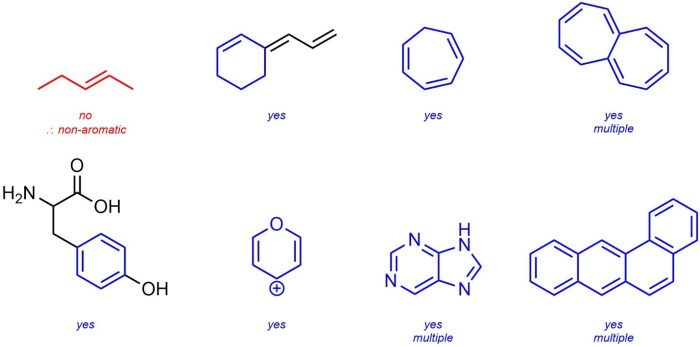
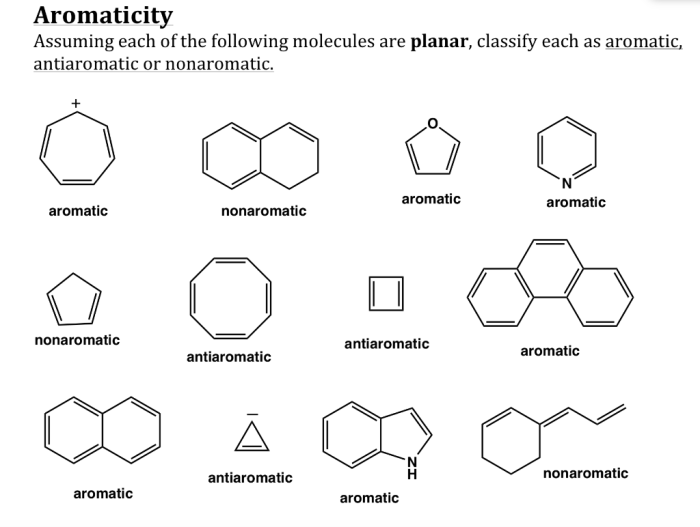
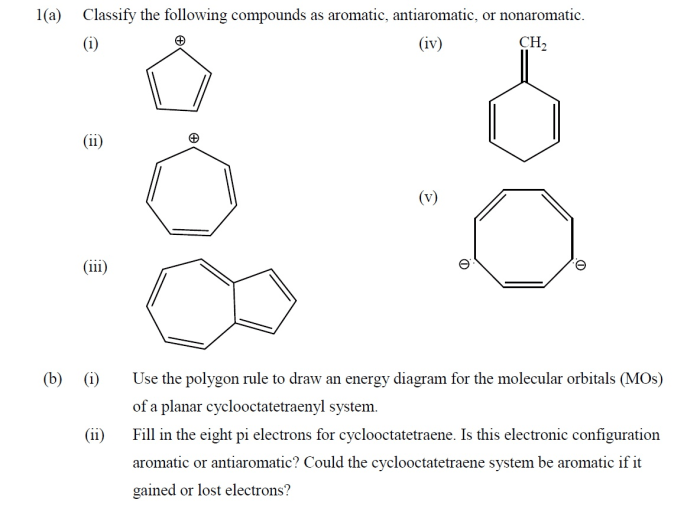
Classify the following compound as aromatic antiaromatic or nonaromatic, the classification of compounds as aromatic, antiaromatic, or nonaromatic is a crucial concept in organic chemistry. Aromatic compounds exhibit unique stability and reactivity due to their cyclic, conjugated π-electron systems. Antiaromatic compounds, on the other hand, are unstable and highly reactive, while nonaromatic compounds lack the characteristic properties of aromatic or antiaromatic compounds.
Hückel’s Rule provides a simple yet powerful tool for classifying compounds based on their aromaticity. This rule states that a compound is aromatic if it contains a continuous ring of (4n + 2) π-electrons, where n is an integer. Antiaromatic compounds have a continuous ring of 4n π-electrons, while nonaromatic compounds do not meet either of these criteria.
1. Introduction: Classify The Following Compound As Aromatic Antiaromatic Or Nonaromatic

In chemistry, compounds can be classified as aromatic, antiaromatic, or nonaromatic based on their electronic structure and properties.
Aromatic compoundsare cyclic, planar molecules with a continuous ring of overlapping p-orbitals. They exhibit high stability and undergo characteristic reactions such as electrophilic aromatic substitution.
Antiaromatic compoundsare also cyclic, planar molecules, but they have a continuous ring of non-overlapping p-orbitals. They are typically highly unstable and reactive.
Nonaromatic compoundsare cyclic molecules that do not meet the criteria for aromaticity or antiaromaticity. They may be planar or non-planar and have varying degrees of stability.
2. Hückel’s Rule
Hückel’s Rule is a simple but powerful tool for predicting the aromaticity of cyclic compounds. It states that a cyclic compound is aromatic if it has:
- A continuous ring of p-orbitals
- A planar structure
- (4n + 2) π-electrons, where n is an integer
Compounds that satisfy Hückel’s Rule are typically aromatic, while those that do not are typically nonaromatic or antiaromatic.
3. Examples of Aromatic Compounds
Some common examples of aromatic compounds include:
- Benzene
- Naphthalene
- Anthracene
- Pyridine
- Furan
These compounds are all cyclic, planar, and have (4n + 2) π-electrons, making them aromatic according to Hückel’s Rule.
4. Examples of Antiaromatic Compounds
Some common examples of antiaromatic compounds include:
- Cyclobutadiene
- Cyclooctatetraene
- p-Benzyne
These compounds are all cyclic, planar, but they do not have (4n + 2) π-electrons, making them antiaromatic according to Hückel’s Rule.
5. Examples of Nonaromatic Compounds
Some common examples of nonaromatic compounds include:
- Cyclohexane
- Cycloheptatriene
- Norbornene
These compounds are either non-cyclic, non-planar, or do not have a continuous ring of p-orbitals, making them nonaromatic.
6. Methods for Classifying Compounds as Aromatic, Antiaromatic, or Nonaromatic
In addition to Hückel’s Rule, there are several other methods that can be used to classify compounds as aromatic, antiaromatic, or nonaromatic. These methods include:
- NMR spectroscopy
- IR spectroscopy
- UV-Vis spectroscopy
- Computational methods
Each of these methods has its own advantages and disadvantages, and the choice of method will depend on the specific compound being studied.
7. Applications of Aromatic Compounds
Aromatic compounds are widely used in a variety of applications, including:
- Medicine:Aromatic compounds are used as drugs, antibiotics, and other pharmaceuticals.
- Materials science:Aromatic compounds are used in the production of plastics, fibers, and other materials.
- Organic chemistry:Aromatic compounds are used as starting materials and reagents in a wide variety of organic reactions.
Common Queries
What is the difference between aromatic and antiaromatic compounds?
Aromatic compounds are stable and have a continuous ring of (4n + 2) π-electrons, while antiaromatic compounds are unstable and have a continuous ring of 4n π-electrons.
How can I use Hückel’s Rule to classify a compound as aromatic, antiaromatic, or nonaromatic?
Count the number of π-electrons in the compound’s conjugated ring system. If the number is (4n + 2), the compound is aromatic. If the number is 4n, the compound is antiaromatic. If the number does not meet either of these criteria, the compound is nonaromatic.
What are some examples of aromatic compounds?
Benzene, naphthalene, and anthracene are all examples of aromatic compounds.